Checklist of NT Vascular Plants
Plant
photos
a change of format
Cycas armstrongii (Miq.)Fam: CYCADACEAE Last updated 20 October 2004 |
|
Cycas armstrongii is one of several of the Family Cycadaceae that can be found in the Top End. These palm-like plants are common in open forest and woodland. The bright green fronds are prominent against a blackened landscape following a bushfire as young fronds emerging from the burnt trunks.
 |
 |
Young fronds arising just towards the end of the 2004 wet season, whilst last year's fronds have turned brown and are falling away. Fronds are smooth, pinnate, 50 to 100 cm long, varying in colour from bright green when young to darker green when matured. Divided into numerous (100 to 180) stiff, straight flat narrow leaflets. 7 to 14 cm long and 0.5 to 0.7 cm wide. Often sharp pointed when mature; soft with velvety hair when young. The fronds are used in the floral industry as backdrop to floral arrangements. (Photo MDR Charles Darwin NP 3/2004) |
An older female Cycas armstrongii with the darker green, harder fronds. (Photo MDR Charles Darwin NP 3/2004) |
|
|
|
The making of a branching cycad. The top has either been damaged by pest or fire with a pair of sprouts shooting from the trunk (Photo MDR Marrara October 2004) |
|

|
|
Detail of the trunk, usually 10 to 15 cm in diameter and most often single stemmed. The grey bark seems reminiscent of crocodile skin in this photo. Often seen blackened by fire. Photo MDR Charles Darwin NP 3/2004) |
Fallen fronds carpet the ground. At the time that the cone develops the usually dark green leaves change to brown and fall from the trunk leaving the apex of the trunk exposed. The cone or seeds can then be clearly seen. New growth arises from the apex as can be seen in the photo to the left. (Photo MDR Darwin 2003) |
|
|
|
Female cycad, pendulous flattened hairy spikes with a triangular tip with sharp spines at the tip and along edges from which the fruit can be seen. Fruit are hard smooth round 2 to 4 cm in diameter becoming red-brown when ripened, containing a single seed. Fruiting March to September. Later in the season often seen fallen around the plant. The nuts can be eaten after grinding them into a flour, baked as a damper, but requires extensive treatment including soaking to remove poisonous constituents. (Photo MDR Charles Darwin NP 3/2004) |
Young female reproductive parts. (Photo Marj King) |
| Cultivation is often difficult. Fresh seed is required but germination may take up to 18 months. C. armstrongii has a preference for sandy well drained soils. Adult specimens may be transplanted with care. This plant however is listed as vulnerable species in the NT. |
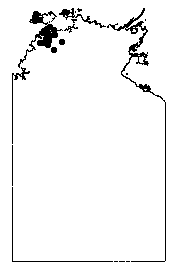
Distribution of C. armstrongii in the NT (from A Draft Management Program For Cycads In The Northern Territory |
Source: Brock J. Top End Native Plants 1988 John Brock. Darwin.
Other Cycas armstrongii information
See the June 2004 TENPS Newsletter for an article on Cycas armstrongii and the impact fire may have on their survival
A Draft Management Program For Cycads In The Northern Territory
(460 kB pdf document)
Cycads are long-lived, slow-growing, woody plants. They have male and female plants, develop cones and reproduce by seed. Sexual dimorphism is evident only in the cone of mature plants with male Cycas bearing a compact conical shaped reproductive structure and female Cycas bearing an open structure which encircles the stem. The age at maturity is unknown for most species and is probably influenced by environmental conditions. In cultivation many cycads reach maturity within 15 years, but it is likely that this time could be considerably longer in the wild. Any attempt to equate age with size is hampered by the fact that plants can regenerate from old bases or root stock.
Reproduction by individual plants can be annual, but may be sporadic or in intervals of up to or over 15 years depending on species and environmental conditions. Cycads are one of the few seed plants that produce motile sperm cells, a sign of their primitiveness. Pollination is believed to be primarily by insect vectors, especially beetles in the family Boganiidae. Cones produce an odour to attract the insects. Seeds are dispersed by gravity, water and animals. Germination may not occur at seed maturity because the embryo usually has an after-ripening period depending on the species. Seed predation by rats and humans can occur. Cycads have various ways to cope with adverse conditions. Most cycads grow in impoverished soils. Additional nitrogen is acquired from cyanobacteria living in symbiosis on the cycad's coralloid roots at the soil surface. Seedlings are vulnerable to predation, fire and desiccation. Contraction of the primary root pulls the apical growing point underground providing protection. If the crown is destroyed, plants will produce new flushes of leaves; and if the apical growing shoots are damaged, branching can occur. In extreme circumstances when part or all of the above ground stem is destroyed, regeneration can occur from stem bases or old root stock.
The management plan also provides a description of all the endemic cycads species in the Northern Territory,
-
Cycas angulata
Cycas armstrongii Miq.
Cycas arnhemica K.D.Hill
Cycas calcicola Maconochie .
Cycas canalis K.D.Hill
Cycas conferta Chirgwin
Cycas maconochiei Chirgwin & K.D.Hill
Cycas orientis K.D.Hill
Cycas pruinosa Maconochie
Macrozamia macdonnellii (F.Muell. ex Miq.) A.DC.
Review of Significant Trade Cycads (CITES November 2003 pdf document)
CITES is the Convention on International Trade in Endangered Species of Wild Fauna and Flora, an international agreement between Governments. Its aim is to ensure that international trade in specimens of wild animals and plants does not threaten their survival.
Cycas armstrongii was named for a gardener from Kew who was a keen collector and resided in the Northern Territory. In areas of the far north of Western Australia and indeed south-west of Darwin near the Western Australia/Northern Territory border it is known as Cycas lanepoolei (C. Gardner). The late J.R. Maconochie stated that this name was synonymous and that C. armstrongii had priority. The form C. lane-poolei extends into the coastal ranges of the top end of Western Australia. Typical of all the family, the plants are dioecious and in this species, also deciduous, generally after April. This cycad grows to approximately 4.0 m and if unburnt caudices can be observed the colour is charcoal grey, very rough and up to 15cm in diameter.
Most mature plants carry on average approximately 50 fronds being a little over 1 m in length, the pinnae pea-green on the surface and mottled with yellow on underside. Each of the adjacent leaf pinnae having prominent midrib vein visible above and below. The male cone emerges from the apex of the plants and is on average 20cm in length, oval but pointed at the apex. The colour is deep rusty hue and composed of hundreds of scales arranged somewhat in a spiral. At first, tightly closed, but opening later to eject dust-like spores.
The female forms a cone at the apex also. The sporophylls enclosing many small ovoid green seed. Later this 'cone' opens fully and the sporophylls arch outward and hang pendant from the plant's crown. Approximately 4 ovules to each sporophyll which is flattened, pubescent and has a triangular spear head shape at the end of each. Thin basal stem lobes are below the ovules The pendant fruit being 20-40cm across and yellowish/brown when ripe. Because of certain familiar aspects this species has earlier been confused with the east coast Cycas media. However C. armstrongii has a much narrower caudex, and Cycas media is seldom deciduous naturally. This particular species along with a sister species Cycas calcicola is much maligned in the territory and suffers from the annual burn off which was part of the aboriginal way of life, but is now continued by many cattlemen across our Top End.
Fires natural or man-produced generally blacken the caudices, and destroy much of the ripe seed. However the resulting new fronds carry a very powerful defence mechanism in the poison Cycasin which affects all the cattlemen's herds. These, as I have stated elsewhere are mostly scrubber, or wild station cattle.
The rejuvenating new fronds of both C. armstrongii and C. calcicola are referred to locally as "fire-fern". C. armstrongii occurs in open forests and generally in sandy loam vicinities. However, in various localities it can be found in dense stands near granite outcrops. Quite often the populations of C. armstrongii spill over into territories where C. calcicola grows and at least one researcher believes he has found obvious hybrids between the two species.
Traditional Botanical Knowledge Peter Lister
Ethnobotany is the use of plants by people, and for indigenous Australians plant uses are numerous. Of the 4500 spp. of plants indigenous to Arnhem Land, around 400 are used by Aboriginal people for food alone. Cycads are an example of plants that are/have been staple carbohydrate sources. They generally fruit in good numbers and the yield can be manipulated (see the section on fire) so as to enable large amounts of food to be prepared and stored and allow gatherings of larger groups for ceremonial purposes. Great care needs to be taken in the preparation of cycad seed as they contain cycasin and other associated glycosides. These accumulative toxins are responsible for the condition known as ‘Guam disease’ - a highly debilitating condition resulting in middle-aged death on the Pacific island of Guam. Aboriginal people in Australia do not suffer with such a condition as the preparation of the seed is rigorous to the point where the toxins are thoroughly removed. This involves regular grinding, washing and finally cooking of the seed paste.
From the The Cycad Pages: Cycas armstrongii
Honouring John Armstrong, a collector for Kew Gardens appointed to establish a government garden at Port Essington in 1838
Historical notes: [C. media forma inermis Miq., Arch. Néerl. Sci. Exact. Nat. 3(5): 235 (1868), in syn. (although Miquel refers to previous publications of forma inermis (Miquel 1842: 7, 17; 1847: 412), this combination was not actually published in these papers, he merely discussed the unusual thornless specimen).
Distinguishing features: distinguished by the glossy green mature new growth and the discolorous leaflets with the midrib equally prominent above and below. This species also has the smallest overall stature of all Australian taxa. Originally distinguished on the basis of the thornless petiole, but petioles are frequently at least partly spinose, and wholly unarmed only on about 10--15% of individuals in a population. This contrasts with C. conferta, which has unarmed petioles in about 90% of individuals in a population. The holotype specimen does not have a complete petiole, but Armstrong 379 does. The petiole of the latter is unarmed, and about 35 cm long, which is outside the usual range for C. armstrongii. The leaflets, however, are well-spaced and match C. armstrongii rather than C. conferta (the spacing is 10--12 mm, whereas that of C. conferta is usually less than 8 mm).
Distribution and habitat: an abundant species, in dense and extensive populations on sand over Tertiary laterites in Eucalyptus miniata - E. tetrodonta forests around Darwin. It extends from the Adelaide River west to the Finniss River and south to the township of Adelaide River, with sporadic occurrences farther south almost to Hayes Creek and in a limited area on Melville Island. Intergrading populations with C. conferta occur at Mt Bundey and near Hayes Creek, and apparent hybrids are known with C. maconochiei subsp. maconochiei.
This species is facultatively deciduous late in the dry season of the monsoonal climatic cycle experienced in its natural habitat. Plants in cultivation or in locally wetter sites can retain leaves for longer, but become dormant, and it appears that the strong seasonal stress is required to rejuvenate plants. Regular (almost annual) dry-season fires experienced over much of the range also promote leaf-drop, and fertile plants are frequently seen with reproductive structure only, and no leaves. The regular fires can also kill the above-ground parts of shorter plants, promoting below-ground branching from an often massive swollen subterranean caudex.
Typification: The type was cited as `In Nova Hollandia boreali ad portum Essington legit Armstrong n. 380 herb. Hookeri.' John Armstrong was a collector for Kew Gardens appointed to establish a government garden at Port Essington in 1838. He then collected in Timor in 1839--45. His collecting ranged beyond the immediate environment of the Port Essington settlement, a number of the plants he collected not being known from that site (including Cycas spp.). C. armstrongii has a limited natural distribution, occurring only in the Darwin district, and the type must have originated from there, considerably to the west of the Port Essington settlement site. Three sheets are present in K, one labelled `No. 379/ Coll. in April 1839 at Port Essington', another labelled `Port Essington/ Australia/ Armstrong', and the third labelled `Port Essington/ (bis)'. The U specimen consists of two leaflets only, but with the rachis cut to match the second of the above K sheets. It appears that the second and third K sheets and the U sheet are all parts of Armstrong 380, and consequently parts of the holotype. Bentham (1873) included C. armstrongii in C. media, and his treatment was followed until Maconochie (1980) recognised the distinctive nature of C. armstrongii, although not on the basis of the unarmed petiole as Miquel had done originally (see below). Although Miquel in the protologue referred to previous publications of forma inermis (Miquel 1842: 7, 17; 1847: 412), this combination was not actually published in these papers, he merely discussed the unusual thornless specimen. This is also an indication that Miquel had seen more than just the U specimen, which has no petiole.
Conservation status: locally extremely abundant, not considered to be at risk. Although not well reserved, the extreme abundance of this species would buffer it from any threat in the medium term. However, frequent fire effectively blocks reproduction, and uncontrolled development progressively alienates significant proportions of the habitat. Several of the Northern Territory species, including this one, are probably the most abundant of all cycads, with populations numbering into tens of millions.
Botanical Description:
Stems arborescent, to 3(-6) m tall, 5-11 cm diam. at narrowest point.
Leaves bright green, semiglossy to highly glossy, 55-90 cm long, slightly keeled to flat (not keeled) in section (opposing leaflets inserted at 130-160° on rachis), with 100-220 leaflets, with white and orange tomentum shedding as leaf expands; rachis usually terminated by a spine. Petiole 10-25(-35) cm long, glabrous, spinescent (usually), for 20-60% of length. Basal leaflets not gradually reducing to spines.
Median leaflets simple, weakly discolorous, 55-140 mm long, 4.5-8 mm wide, inserted at 70-90° to rachis, decurrent for 3-5 mm, narrowed to 3.5-5 mm at base (to 50-85% of maximum width), 6-14 mm apart on rachis; median leaflets section flat; margins flat; apex aristate, not spinescent; midrib raised above, raised below.
Cataphylls linear, pungent, pilose, persistent.
Pollen cones ovoid, orange, 11-20 cm long, 7.5-10 cm diam.; microsporophyll lamina firm, not dorsiventrally thickened, 25-35 mm long, 14-17 mm wide, fertile zone 24-28 mm long, sterile apex 4-6 mm long, level, apical spine prominent, sharply upturned, 7-10 mm long.
Megasporophylls 13-22 cm long, grey-tomentose and brown-tomentose; ovules 2-4, glabrous; lamina lanceolate, 30-70 mm long, 18-35 mm wide, regularly dentate, with 20-28 pungent lateral spines 1-4 mm long, 1-2 mm wide, apical spine distinct from lateral spines, 10-25 mm long.
Seeds flattened-ovoid, 34-37 mm long, 32-36 mm wide; sarcotesta orange-brown, not pruinose, 3-4 mm thick; fibrous layer absent; sclerotesta smooth. Spongy endocarp absent.]
Andrew R. Watkinson A1 and Jane C. Powell A1 The life history and population structure of Cycas armstrongii in monsoonal northern Australia. Oecologia 1997: 111 (3): 341-349
A1 Schools of Environmental and Biological Sciences, University of East Anglia, Norwich, NR4 7TJ, UK Fax: (+44) 01603 592250; e-mail:a.watkinson@uea.ac.uk
Abstract: The cycad Cycas armstrongii is endemic to the north-western corner of the Northern Territory in Australia. Here we provide data on its life history and population structure from four populations across its range. Few plants reproduced before they were 1 m in height. There was considerable variation in the proportion of reproductive plants between sites and years, but the sex ratio in all populations was close to 1:1. The growth rate of plants was approximately 4.5 cm yearm1 which indicates that most plants are less than 100 years old and that the tallest individuals are likely to be little older than this. The annual fecundity of female plants ranged from 12 to 80 seeds; there was no evidence that fecundity varied with plant height. Dispersal was restricted generally to less than 1 m and the distribution of dispersal distances was fitted well by a gamma distribution. Recruitment occurred through both seedlings and vegetative sprouts and the proportion of juveniles (plants without trunks) in the populations varied between one-quarter and two-thirds. The data indicate that recruitment is episodic, but occurs more frequently under the current fire regime than amongst the canopy trees. It is shown that there is considerable variation in the dynamics of populations between sites and that the long term dynamics of a population cannot readily be inferred from an examination of the size structure at a single point in time.
Return to Home page